Expert Commentary
Thank you to Doctors Richardson and Neill on this excellent, succinct summary of the challenges we face in the ED with inpatient boarding. I would like to highlight a few key themes and summarize some thoughts.
Words Matter
Firstly, I would offer that words in this context matter, and the way we frame this message impacts our ability to create our “burning platform” (1), foster buy-in, and ignite change. As many emergency physicians and hospital leaders can attest, often the most difficult step in improving ED crowding and inpatient boarding is to create a unified vision with shared goals. In that respect, this is not “ED boarding” but rather hospital or inpatient boarding leading to ED crowding. The verbiage of “ED boarding” creates the connotation that it is an ED problem and only up to us to solve. Rather, and more accurately, it is inpatient boarding in the ED, leading to ED crowding. Illustratively, a 2009 Government Accountability Office (GAO) report confirmed that the most important cause of ED crowding is the lack of access to inpatient beds (2). How we message this to leaders, create this burning platform, and speak to this concept with colleagues, learners, and patients is a purposeful choice to create this unified vision.
What’s the Current State?
The most recent data from the Emergency Department Benchmarking Alliance (EDBA) (3) demonstrates the following aggregate numbers for ED’s similar to NMH (80-100k visits):
Median length of stay for all ED patients 246 minutes
4.4% left without being seen (LWBS) – about ~3500 patients annually for 80k annual volume ED) (NB: LWBS predictably increases in a linear fashion as ED waiting room time increases )
We are seeing more patients that are older, higher acuity, and subsequently receive more ED testing.
65% of hospital admissions come through the ED (compared to 10% direct admissions, 8% transfers, and 15% L&D)
Median boarding time 169 minutes (41% of ED LOS for admitted patients is boarding time)
As was mentioned, increased duration and incidence of boarding is associated with urban high-volume EDs as well as in those patients who arrive by EMS, during office hours, are older, and receive advanced imaging (2). Longer boarding time is associated with higher volumes, acuity, and admission rates; longer hospital lengths of stay, and being seen by a resident or intern (2).
A critical framework to consider in this current state is the possible return on investment for various solutions. One adage is that “the cheapest hospital bed is the one in the ED hallway,” which gets at the concept that boarding in the ED is so prevalent because hospitals maximize revenue by prioritizing non-ED admissions at the expense of caring for inpatients in ED hallways. One study (4) looked at this, using a high volume urban academic hospital with a typical revenue of non-ED admissions double that of ED admissions, and the authors found that by reducing boarding time by 1 hr it would result in $9k-$13k additional daily revenue from capturing LWBS and diverted patients. To meet this additional ED demand, dynamic bed management policies were simulated, and the optimal strategy that reduces ED boarding time, LWBS, and diverted patients, increasing ED arrivals, and optimizing non-ED admissions would generate an additional $2.7 to $3.6M annual revenue.
Boarding Effects on Patients
Why such a fuss? Other than lack of control over the clinical environment being a leading driver of burnout among physicians, (5) EM near the top with respect to prevalence of burnout, (6) and burnout contributing to detrimental patient-centered outcomes, there are additional patient-centered outcomes that are directly impacted – negatively – by inpatient boarding in the ED.
If you take nothing else away from this topic, here is the punchline:
Boarding inpatients in the ED causes care delays, adverse outcomes across a variety of conditions,7 increased medication errors, (8) increasing rates of delirium, (9) worsens door to doctor time, increases ED LOS for all ED patients, increases inpatient LOS, and worsens hospital mortality.10 Put another way – boarding inpatients in the ED causes death.
Solutions
Alas, it is not all doom and gloom. Solutions are many but variable in use and impact. As many would offer, change starts with us. ED presence and leadership in these discussions and initiatives is a necessity. Leading with persistence, effective communication, advocacy, empathy, and the ability to tell a story that is patient-oriented to drive change and create our burning platform is a must.
One study (11) found that no single strategy was consistently effective at alleviating hospital boarding and ED crowding. Rather, four broad organizational characteristics were associated with better ED performance – a direct surrogate for hospital performance – senior executive involvement, hospital-wide strategies, data-driven management, and performance accountability. In high performing hospitals, executives identified crowding as a top priority, clearly articulated performance goals, provided resources, and had leadership on the floor to monitor performance.
Further, researchers at one community ED in the Kaiser system found that a hospital leadership-based program aimed at reducing admit wait times was associated with a significant decrease in boarding time, ED LOS, LWBS rate, and ambulance diversion as well as an increase in patient experience (12).
More strategically speaking, surgical schedule smoothing has been shown to significantly impact boarding times and ED crowding, among a bevy of other financial and operational metrics (13-14).
Full capacity protocols (FCPs), such as Beds in Progress (BIP) has demonstrated safety, success, and satisfaction (15). Patients prefer to board in the inpatient hallway rather than the ED, and yet only 20% of hospitals have successfully implemented this strategy (16-17).
ED Admissions, Hospital Discharges, and Flow
Strategies to optimize a variety of constraints in this process are numerous and often innovative, including interventions such as discharges by 10 or 11, post acute care preferred provider networks to facilitate disposition in those who require advanced rehab or nursing services, multidisciplinary outpatient pathways (low risk chest pain or TIA, AFib, VTE, pneumonia, sickle cell), community paramedicine, health system capacity alignment utilizing command centers and throughput committees, and optimizing demand-capacity alignment in the inpatient setting (timely and effective consults, procedures, tests, etc). All have shown varying degrees of impact, safety, and success in improving hospital boarding and ED crowding (10,18).
Fixing Our Shop First
In the end, successful physician leaders have demonstrated excellent clinical acumen as well as a track record of leadership within their own environment. Effective change management, engagement, and creation of a “burning platform” will fall on deaf ears unless we demonstrate an endless desire, effort, and dedication towards optimizing ED operations. Successful strategies, endorsed by national organizations such as IHI or ACEP and grounded in LEAN thinking, include those as you have mentioned above including: direct bedding with bedside triage and registration, separating flows such as fast tracks, super tracks, vertical 3’s, or split flow models, provider in triage (PIT), CDU creation and optimization, and aligning demand-capacity relationships can all effect powerful change, improve ED LOS, decreased LWBS, decrease wait times, and improve patient and staff experience.
Strategies to Avoid
Framing this issue as ED Boarding, and thus an ED problem that is up to the ED to solve, is often a short-sighted and limited perspective. Placing the blame entirely on the ED, or even the patients who choose to utilize the ED, can create winds of change around initiatives with little or no impact such as diverting low acuity patients, financially disincentivizing care ambulance diversion or increasing ED bed capacity.19
Thank you again to Doctors Richardson and Neill for summarizing a topic of perhaps the most importance to emergency physicians, as we attempt to drive change around the concepts of flow, boarding, and crowding for the safety and satisfaction of ourselves and our patients.
Literature Cited:
Guarisco J. Cracking the code: fixing the crowded emergency department, part 1 –building the burning platform. CommonSense 2013;September/October. 18-20. https://www.aaem.org/resources/publications/common-sense/right-column-items/cracking-the-code/archive
Pitts SR, Vaughns FL, Gautreau MA, Cogdell MW, & Maisel Z. A cross-sectional study of emergency department boarding practices in the United States. Acad Emerg Med. 2014;21:497-503.
ED Benchmarking Alliance. 2018 Emergency Department Performance Measures. https://www.edbenchmarking.org/
Pines JM, Batt RJ, Hilton JA, & Terwiesch C. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011;58:331-340.
West CP, Dyrbye LN, Shanafelt TD. Phyisican burnout: contributors, consequences and solutions. J Intern Med. 2018;283(6):516-529.
Medscape National Physician Burnout, Depression, and Suicide Report 2019.
Pines JM, Pollack CV, Diercks DB, Chang AM, Shofer FS, & Hollander JE. Association between emergency department crowding and adverse cardiovascular outcomes in patients with chest pain. Acad Emerg Med. 2009;16:617-625.
Kulstad EB, Sikka R, Sweis RT, Kelley KM, & Rzechula KH. ED overcrowding is associated with an increased frequency of medication errors. AJEM.2010;28:304-309.
Singla A, Sinvani L, Kubiak J, Calandrella C, Brave M, Li T, et al. Emergency department hallway bed time is associated with increased hospital delirium. Ann Emerg Med. 2019;74(4S):S33.
Morley C, Unwin M, Peterson GM, Stankovich J, & Kinsman L. Emergency department crowding: a systematic review of causes, consequences, and solutions. PLoS ONE. 2018;13(8):e0203316.
Chang AM, Cohen DJ, Lin A, Augustine J, Handel DA, Howell E, et al. Hospital strategies for reducing emergency department crowding: a mixed-methods study. Ann Emerg Med. 2018;71:497-505.
Patel PB, Combs MA, & Vinson DR. Reduction of admit wait times: the effect of a leadership-based program. Acad Emerg Med. 2014;21:266-273.
Litvak E. Innovations: surgical smoothing. Urgent Matters. https://smhs.gwu.edu/urgentmatters/news/innovations-surgical-smoothing
Ryckman FC, Adler E, Anneken AM, Bedinghaus CA, Clayton PJ, Hays KR, et al. Cincinnati Children’s Hospital Medical Center: redesigning perioperative flow using operations management tools to improve access and safety. In Managing Patient Flow in Hospitals: Strategies and Solutions. 2nd ed. 97-111.
Vicellio A, Santora C, Singer AJ, Thode HC, Henry MC. The association between transfer of emergency department boarders to inpatient hallways and mortality: a 4-year experience. Ann Emerg Med. 2009;54:487-491.
Garson C, Hollander JE, Rhodes KV, Shofer FS, Baxt WG, & Pines JM. Emergency department patient preferences for boarding locations when hospitals are at full capacity. Ann Emerg Med. 2008;51:9-12.
Vicellio P, Zito JA, Savage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding. J Emerg Med. 2013;45(6):942-946.
ACEP EM Practice Committee. 2016. Emergency department crowding: high impact solutions.
Han JH, Zhou C, France DJ, Zhong S, Jones I, Storrow AB, et al. The effect of emergency department expansion on emergency department overcrowding. Acad Emerg Med. 2007;14:338-343.
















![Summary of the Pathophysiology of Heat Stroke [1]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785361684-SVDHAE847O881FLLW493/Picture1.png)
![Subject in a cold water-immersion bath after heat- stroke [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785609566-OWN8IJYW5Y4QGFO2YPWI/Picture2.png)
![Evaporative and conductive cooling methods--note the placement of ice packs in axilla, groin as well as the cooling fan overhead [4]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785678671-8PWD8653TJQ61EQ1F6VS/Picture3.png)
![table of cooling methods [6]](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593785810930-ZUZ2S1CGXXO1ME2854ZJ/Picture4.png)
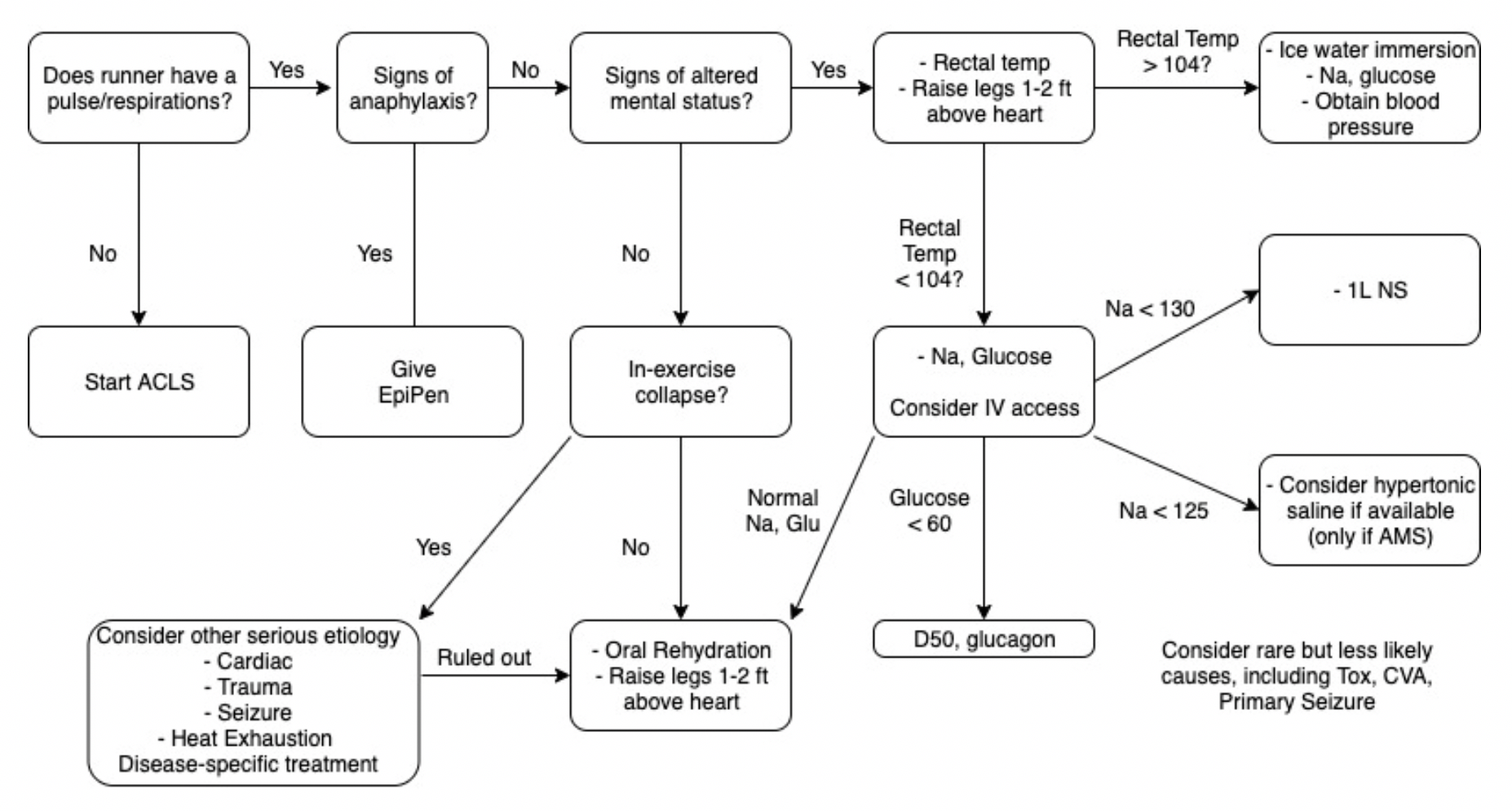
![Collapse_Algorithm[1] (1) (1).png](https://images.squarespace-cdn.com/content/v1/549b0d5fe4b031a76584e558/1593786307567-LB1EFX5KMHS371YBFKMZ/Collapse_Algorithm%5B1%5D+%281%29+%281%29.png)