Written by: David Feiger, MD (NUEM ‘22) Edited by: Abiye Ibiebele, MD (NUEM ‘21) Expert Commentary by: Daniel Schimmel, MD, MS
Introduction
An emergent pericardiocentesis may be a life-saving procedure and is indicated in patients with pericardial effusion and associated hemodynamic instability. The degree of pericardial effusion severity lies on a continuum and when associated with hemodynamic instability, is known as cardiac tamponade. The volume and rate at which the effusion develops most affects a patient’s hemodynamics. The clinical exam findings – hypotension, distant heart sounds, and jugular venous distention (known as Beck’s Triad) – often do not occur simultaneously if cardiac tamponade is suspected and confirmed early.
Given the urgency of the situation and severity of the patient’s clinical status, blind insertion of a needle into the pericardial space using anatomic landmarks has historically been the method of choice to restore hemodynamic stability. The availability of bedside ultrasound in many emergency departments more recently has led many physicians to pursue an image-guided pericardiocentesis. Various studies have demonstrated decreased mortality and morbidity with an ultrasound-guided approach when compared to a blind approach. However, depending on the clinical context and tools available, a blind approach may be the optimal choice.
Indications and Contraindications
Hemodynamic instability secondary to pericardial effusion, is the number one reason to perform an emergent pericardiocentesis in the emergency room. Pericardiocentesis for patients with symptomatic pericardial effusion but without hemodynamic instability may be deferred to inpatient management.
An emergent pericardiocentesis has few contraindications. Aortic dissection or cardiac free wall rupture is sometimes considered an absolute contraindication to pericardiocentesis, but in the absence of immediately available life-saving procedures, pericardiocentesis should be strongly considered. Relative contraindications include use of anticoagulation, platelets < 50K, and uncorrected coagulopathy. Furthermore, trauma patients with hemopericardium should preferentially undergo surgical pericardial drainage or emergency thoracotomy.
Blind Emergent Pericardiocentesis
Relevant Anatomy to Keep in Mind
Internal thoracic artery (internal mammary artery) – artery running cephalad to caudal on the anterior chest wall parallel to the sternum bilaterally
Neurovascular bundle – a collection of an intercostal vein, artery, and nerve running caudal to each rib
Materials
Sterile gloves, gown
Chlorhexidine swab
At least a 7 cm 18-gauge spinal needle or introducer needle if planning for continuous access to pericardial space
Syringes (10mL and 60-80mL)
Three-way stopcock
Plastic drainage tubing
Surgical clamp (optional)
1) Position the patient appropriately.
Provide respiratory support with nasal cannula or mechanical ventilation as indicated. Placing patients upright at 30 degrees to enhances comfort and allows gravity-dependent pooling of pericardial fluid.
2) Select an entry site.
Left parasternal and apical approaches are the most commonly pursued and have been shown to be superior to the classic subxiphoid approach in observational studies. When selecting an entry point, recall the location of the internal thoracic artery and subcostal neurovascular bundle. Cleanse a large area of the chest and upper abdomen with the chlorhexidine swab.
3) Insert and advance the spinal needle.
Insert the spinal needle into skin. Once the bevel is in the skin, remove the stylet, attach a three-way stopcock with a large volume syringe attached and maintain negative pressure as you advance the needle. Avoid sliding the needle laterally to prevent lacerating tissues. If a different trajectory is required, retract the needle, keeping the bevel in the skin and drive the needle at the desired angle maintaining negative pressure on the syringe. Momentary resistance may be met as the needle approaches the pericardium, but with continued advancement, a “pop” may be felt followed by aspirate in the syringe and improving patient hemodynamics. See below for more detailed instructions for each approach.
Subxiphoid
Insertion: 1 cm inferior to the left xiphocostal angle, 30 degrees with the patient’s chest
Direction: Towards left mid-clavicle. If unsuccessful, retract the spinal needle and redirect 10 degrees towards the patient’s right
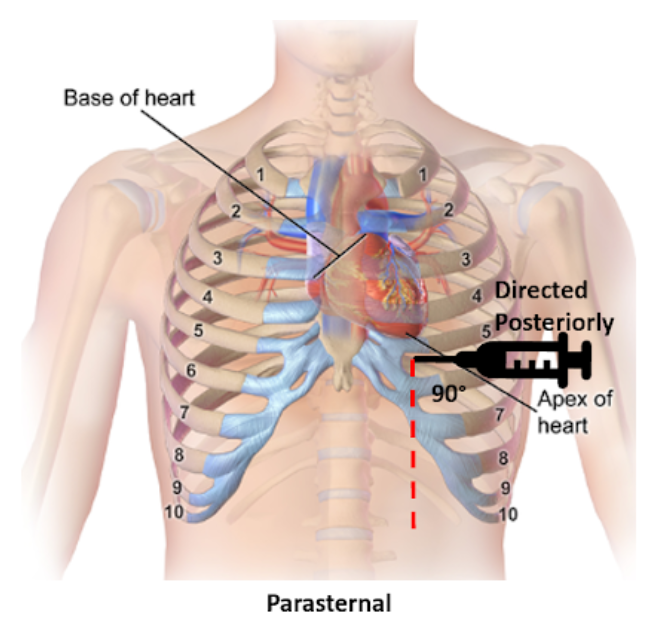
Parasternal
Insertion: Fifth intercostal space at the left parasternal border no more than 1 cm lateral, cephalad to the inferior rib and perpendicular to the patient’s chest
Direction: Posterior
Apical
Insertion: Fifth, sixth, or seventh left intercostal space approximately 6 cm from the parasternal border, cephalad to the inferior rib
Direction: Patient’s right shoulder
4) Draining the pericardial effusion.
Steady the needle with a surgical clamp at the needle shaft closest to the surface of the skin effectively preventing further needle advancement. Attach the plastic tubing to the stopcock allowing emptying of the syringe contents into a collecting vestibule without exchanging syringes. If planning to place a more permanent line (see “Establishing Continuous Access to the Pericardial Space”), consider aspirating just enough fluid to stabilize the patient’s hemodynamics and leave the remaining pericardial fluid to provide space for placing a line.
Tips for Pericardiocentesis with Ultrasound Guidance
Additional materials:
Bedside ultrasound
Sterile ultrasound probe cover
Skin marker
The safety and success of the steps above can be enhanced with bedside ultrasound. Bedside ultrasound can help prior to the procedure by finding the largest effusion nearest the skin and during the procedure by visualizing the needle trajectory in order to avoid important organs and other structures. There are several methods of ultrasound use in pericardiocentesis.
In static guidance, ultrasound is only used for procedure planning. The subxiphoid, parasternal, and apical views can be explored to find the largest effusion and determine the optimal entry. Often, the needle entry point is marked with a skin marker and another mark is made for the planned trajectory. Distance and angle from the skin to the effusion is also noted.
In dynamic guidance, the needle is passed through the skin parallel to the 2D plane created by the ultrasound probe after finding an effusion pocket. The needle can be visualized as it is advanced towards the pericardial effusion and enters the pericardial space.
No ultrasound? Hook up an EKG!
Additional materials:
Continuous EKG monitor
Wire with alligator clips
A continuous EKG can be used to prevent inadvertent traversing of the myocardium with the needle without an ultrasound. Attach one alligator clip to the needle and the other to an anterior lead on a continuous EKG. ST-elevations will be apparent on the EKG if the myocardium is touched. If ST-elevations are noted, simply retract the needle.
Confirmation of Pericardial Access with Ultrasound Guidance
Additional materials:
Bedside ultrasound
Two 10 mL syringes (one with 4 mL of saline, one with 0.5 mL of air)
Confirming success in accessing the pericardial space can also be made injecting agitated saline and visualizing bubble artifact on ultrasound. To do so, attach the two saline syringes to the three-way stopcock. Turn off access towards the patient and rapidly push the contents from one syringe to the other until the fluid appears opacified. When all the saline is in one syringe, close off the access to the empty syringe and push the fluid towards the patient, visualizing it on the ultrasound. Confirmation is especially important when blood is aspirated and helps distinguish between pericardial versus ventricular placement.
Establishing Continuous Access to the Pericardial Space
Additional materials:
Flexible or curved-tip (J) guidewire
6-8 Fr drainage catheter (pigtail, sheath, or central venous catheter)
7 cm or longer 18-gauge introducer needle (as opposed to spinal needle)
Dilator
11 blade scalpel
Suture
Needle driver
Many of the materials above may be found in a central venous kit. Using the Seldinger technique, a line can be placed for continuous access to the pericardial space. Ensure that an introducer needle is used when initially accessing the pericardial space. Keeping the surgical clamp and needle in place, remove the stopcock and syringe, and gently advance the guidewire just beyond the bevel of the needle. Remove the introducer needle, ensuring the guidewire does not move, and use the scalpel to make a short incision at the guidewire’s entry into the skin. Advance the dilator over the guidewire to loosen the tissue. Remove the dilator leaving the guidewire in place and advance the drainage catheter just 1 cm beyond the guidewire into the pericardial space. Retract the guidewire while maintaining the position of the catheter, aspirate fluid to confirm placement, and secure the drain’s position with sutures and placement of a sterile dressing. Further confirmation of proper placement can be made using the agitated bubble study as described in “Confirmation of Pericardial Access with Ultrasound” above.
Conclusion
There are very few contraindications for an emergent pericardiocentesis in a patient with pericardial effusion and hemodynamic instability. While ultrasound-guided pericardiocentesis have lower morbidity and mortality rates, clinical context and emergent patient decompensation may make an image-guided procedure infeasible. Apical and parasternal access with a blind procedure have fewer complications than a subxiphoid approach. A pericardiocentesis may be a life-saving intervention as even a small amount of fluid aspirated may dramatically improve a patient’s hemodynamics.
Expert Commentary
Thank you Dr. Feiger for this excellent summary of pericardiocentesis. From center to center there can be variability in the expertise and mechanism through which pericardiocentesis is performed. Some institutions may have an echo focused pericardiocentesis service, while some institutions may perform the bulk of their pericardiocentesis in an interventional suite with the assistance of fluoroscopic imaging. However, there are times when pericardiocentesis must be performed as an emergency procedure with landmark guidance. Luckily, point of care ultrasound has been very commonplace in the emergency department and intensive care units facilitating visualization of fluid pockets that can be identified for safer access and to demonstrate successful drainage at the end of the procedure.
The relevant anatomy and associated complications from tissue injury during needle advancement for pericardiocentesis changes depending on the planned access route.
Subcostal
Liver laceration or puncture
Pneumothorax
Right atrial or ventricular laceration
Apical
Pneumothorax
Left or right ventricular laceration
Parasternal
Pneumothorax
Right ventricular laceration
In each location, careful use of ultrasound can avoid potential life-threatening complications. How the ultrasound is used also varies depending on location. Generally the ultrasound can be used to identify a path and I will have trainees hold the ultrasound the exact same way as they would hold their needle to mimic the path they will use when advancing into the pericardial space.
Common errors that I have seen are listed below.
Moving the needle side to side while it is in the body to try and find the appropriate space. I am impressed at the body’s ability to tolerate a straight in and out movement of a needle. But moving a needle tip back and forth creates lacerations that are difficult to heal and may result in tissue damage and uncontrollable bleeding requiring surgical intervention.
Moving the needle to find it under ultrasound, rather than moving the probe to find the needle can be dangerous. If the needle is in the wrong location, it should be moved. Otherwise, do not bounce the needle within the body to try and identify it on ultrasound.
A common subcostal error is needle path facing towards the spine through the torso. The subcostal position can be more successful with the patient upright at 30 degrees so that the fluid layers to the bottom portion of the heart, increasing the pocket size for needle entry. However, this position then requires the physician to aim slightly up, almost moving parallel to the ribs, to avoid needle entry being too low and passing underneath the effusion.
Also from the subcostal position, the initial position angle for subcostal pericardiocentesis should be to the left middle cervical bone as Dr. Feiger mentioned. However, many performing subcostal pericardiocentesis overcompensate initially and head too laterally to capture fluid.
From the apical position, the fluid is likely best obtained with the patient in the left lateral decubitus position. A drop door that is often present on an sonographer’s bed for performing echocardiograms is nice but not necessary.
As mentioned, the cardiac probe is not generally used to watch needle entry but to identify the most optimal path. However, if also equipped with a vascular probe, in the parasternal location, the vascular probe can easily watch the needle enter into the pericardial space while avoiding delicate structures like the internal thoracic artery.
Using fluoroscopic guidance, needle location can be identified in relation to the movement of the cardiac border and wire advancement can be used to identify a course consistent with the pericardial space and not limited by other cardiac structures. A small amount of contrast can be injected into the space and seen to highlight the cardiac borders allowing confirmation of the pericardial space.
But in the absence of fluoroscopy, and a more specific confirmation, an echo with agitated bubbles injected can verify presence of the needle, or a microcatheter, in the pericardial space. If after injection, bubbles are seen within the cardiac chambers, the needle should be withdrawn. If possible to obtain a pressure measurement during the procedure, this is one other guide to inform the operator of the needles location. If a right ventricular waveform Is present, the needle has entered the ventricular space. It may be possible to withdraw the needle until the high pressures of the ventricle reduce and if the needle aspirates, another agitated bubble injection can be performed.
An urgent pericardiocentesis with a large effusion can be easily performed at the bedside, particularly with the aid of an ultrasound and knowing the anatomy with immediate improvement in hemodynamics. Send the fluid for analysis and exchange the needle for a drain to ensure patient stability until the evaluation is complete.
Daniel Schimmel, MD, MS
Interventional Cardiologist
Northwestern Memorial Hospital
Associate Professor
Feinberg School of Medicine
How To Cite This Post:
[Peer-Reviewed, Web Publication] Feiger, D. Ibiebele, A. (2021, Feb 8). Pericardiocentesis. [NUEM Blog. Expert Commentary by Schimmel, D]. Retrieved from http://www.nuemblog.com/blog/pericardiocentesis.
Other Posts You May Enjoy
References
Gueria, Rajesh. “Ultrasound Guided Procedures in Emergency Medicine Practice - Pericardiocentesis.” Sonoguide, 2008, www.acep.org/sonoguide/pericardiocentesis.html.
Heffner, Alan C. “Emergency Pericardiocentesis.” Edited by Allan B Wolfson et al., UpToDate, 29 May 2019, www.uptodate.com/contents/emergency-pericardiocentesis?search=pericardiocentesis&source=search_result&selectedTitle=1~65&usage_type=default&display_rank=1.
Konnoff-Phillips, Kelly and Janis Provinse, directors. Agitated Saline Bubble Study. Highland Hospital Emergency Department, 2015.
Maisch, Bernhard, and Sabine Pankuweit. Interventional Pericardiology: Pericardiocentesis, Pericardioscopy, Pericardial Biopsy, Balloon Pericardiotomy, and Intrapericardial Therapy. Springer, 2011.
Nicks, Bret A, et al. Emergency Pericardiocentesis. New England Journal of Medicine, 22 Mar. 2012, www.nejm.org/doi/full/10.1056/NEJMvcm0907841.