Written by: Patrick King, MD (NUEM ‘23) Edited by: Adesuwa Akehtuamhen, MD (NUEM ‘21)
Expert Commentary by: Matt McCauley, MD (NUEM ‘21)
Expert Commentary
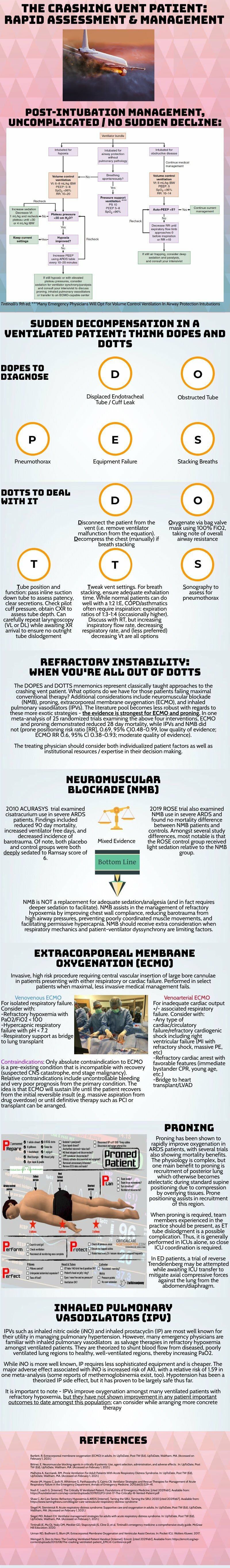
Thank you for this succinct summary of an incredibly important topic. We as emergency physicians spend a lot of time thinking about peri-intubation physiology but the challenges do not end once the plastic is through the cords. The frequency with which our ventilated patients stay with us in the ED has been increasing for years and will likely continue to do so1. This means that managing both acute decompensation and refractory hypoxemia needs to be in our wheelhouse.
The crashing patient on the ventilator can be truly frightening and your post effectively outlines a classic cognitive forcing strategy for managing these emergencies. A truism in resuscitation is to always rule out the easily correctable causes immediately. In this case, it means removing the complexity of the ventilator and making things as idiot-proof as possible. Once you’ve ruled out the life threats like pneumothorax, tube displacement, and vent malfunction, you can try to bring their sats up by bagging. Just make sure that you have an appropriately adjusted PEEP valve attached to your BVM for your ARDS patients; the patient who was just requiring a PEEP of 15 isn’t going to improve with you bagging away with a PEEP of 5.
Once you’ve gotten the sats up and the patient back on the vent, your ventilator display can provide you with further data as to why your patient decompensated. Does the flow waveform fail to reach zero suggesting breath stacking and a need for a prolonged expiratory time? Is the measured respiratory rate much higher than your set rate with multiple breaths in a row indicating double-triggering? The measured tidal volume might fall short of your set tidal volume. This points towards a circuit leak, cuff leak, or broncho-pleural fistula. Maybe you’re seeing the pressure wave dip below zero mid-inspiration and the patient is telling you that they are in need of faster flow, a bigger breath, or deeper sedation. In these situations, your respiratory therapist is going to be your best friend in managing this patient-ventilator interactions2.
As your post alludes to, sometimes patients remain hypoxemic despite our usual efforts and refractory hypoxemia can be an intimidating beast when you’ve got a busy ED burning down around you. If your cursory efforts to maintain vent synchrony by playing with the ventilator dials have failed, there’s no shame in deepening sedation which will work to decrease oxygen consumption and prevent derecruitment. Once sedated, work with your RT to find appropriate PEEP and tidal volumes to meet your goals.
Most patients can be managed with usual lung-protective ventilation but some patients will require more support and you’ve correctly identified several salvage therapies. My general approach is to pursue prone positioning in any patient with a P:F ratio approaching 150 despite optimal vent settings as it has the only strong mortality benefit of the therapies outlined above. Proning in the ED is resource intensive and is probably better pursued as a department-wide protocol rather than you and your charge nurse trying to figure it out in the middle of the night3.
As you’ve pointed out, the neuromuscular blockade has more limited evidence and is not required for prone ventilation. Upstairs, we accomplish this with continuous infusions but in the ED you may be more comfortable using intermittent boluses of intubation dose rocuronium. Just make sure your patient is unarousable. I reach for this if I’m unable to achieve ventilator synchrony with sedation alone as it allows for very low tidal volumes and inverse ratio ventilation. I see inhaled pulmonary vasodilators in a similar light: there’s no data on patient-oriented outcomes but they can make your numbers look prettier while you wait for more definitive interventions such as transfer.
This finally brings me to VV ECMO for refractory hypoxemia. It’s worth considering that while there is some evidence for a mortality benefit for ECMO in ARDS, the evidence base is mixed. The CESAR trial did show a mortality benefit in patients transferred to an ECMO center but only 76% of patients actually received ECMO upon transfer4. The larger and more recent EOLIA trial failed to demonstrate this improvement in mortality5. The conclusion I take from this is that treatment at a high volume center matters and that a boarding patient with refractory hypoxemia warrants an early consideration for transfer to a tertiary center if high-quality ARDS care can’t be accomplished upstairs at your shop.
References
Mohr NM, Wessman BT, Bassin B, et al. Boarding of Critically Ill Patients in the Emergency Department. Crit Care Med. 2020;48(8):1180-1187. doi:10.1097/CCM.0000000000004385
Sottile PD, Albers D, Smith BJ, Moss MM. Ventilator dyssynchrony – Detection, pathophysiology, and clinical relevance: A Narrative review. Ann Thorac Med. 2020;15(4):190. doi:10.4103/atm.ATM_63_20
McGurk K, Riveros T, Johnson N, Dyer S. A primer on proning in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(6):1703-1708. doi:10.1002/emp2.12175
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet Lond Engl. 2009;374(9698):1351-1363. doi:10.1016/S0140-6736(09)61069-2
Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. Published online May 23, 2018. doi:10.1056/NEJMoa1800385
Matt McCauley, MD
How To Cite This Post:
[Peer-Reviewed, Web Publication] King, P. Akehtuamhen, A. (2022, Feb 28). Crashing Ventilator Patient. [NUEM Blog. Expert Commentary by McCauley, M]. Retrieved from http://www.nuemblog.com/blog/crashing -vent-patient.