Written by: Brett Cohen, MD (NUEM PGY-3) Edited by: Duncan Wilson, MD (NUEM Alum ‘18) Expert commentary by: Luisa Morales-Nebreda, MD
Expert Commentary
I’ll start by saying that the assessment of a patient’s intravascular volume status and fluid responsiveness is one of the most difficult tasks in clinical medicine, yet it remains a crucial one to adequately manage acute circulatory failure.
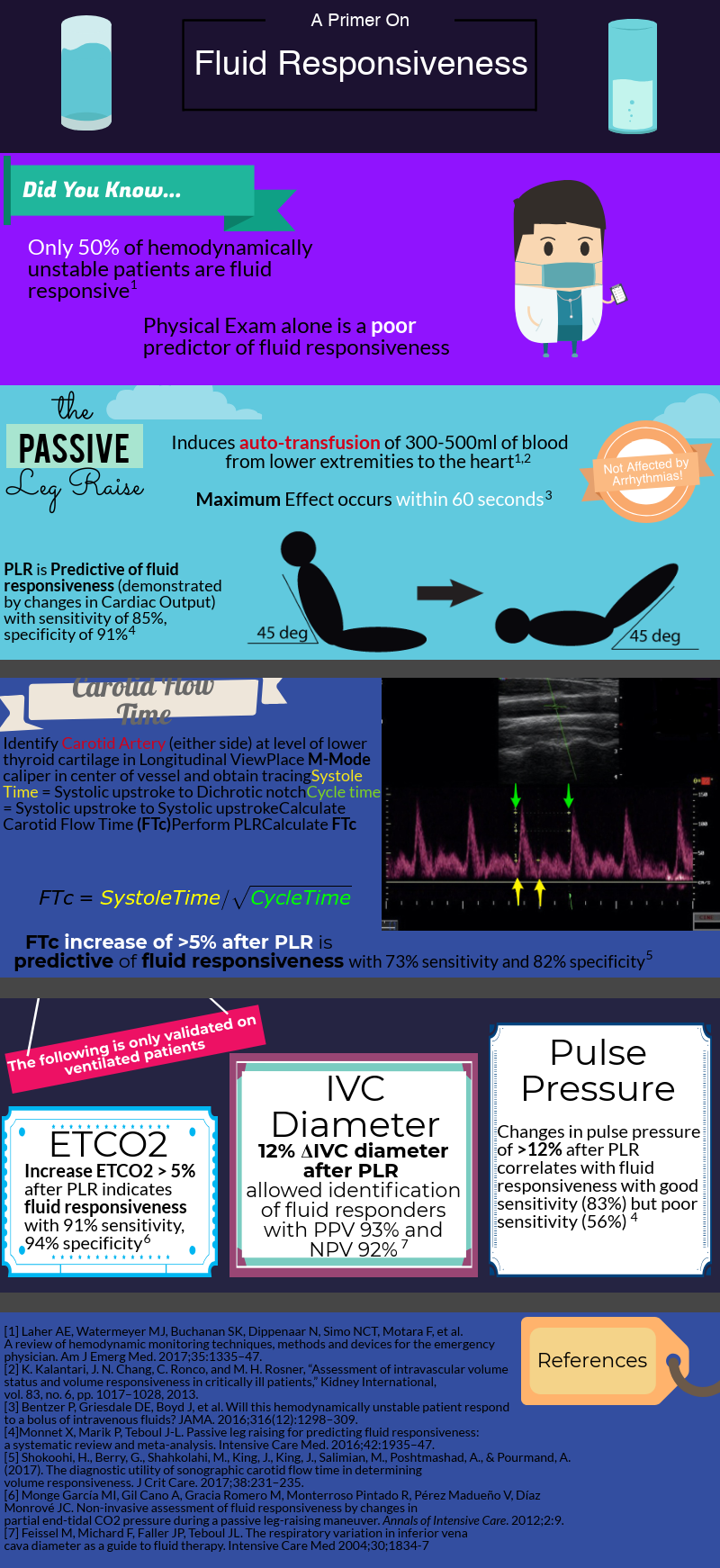
After decades of dedicated research to the critically ill, we’ve learned that rigid protocols lead to large amounts of fluid administration, and that fluid overload is associated with increased morbidity and mortality in septic shock and ARDS patients. As you mentioned, only half of hemodynamically unstable patients are fluid responsive, prompting clinicians to describe novel tools/methods to better evaluate fluid responsiveness.
How do we define fluid responsiveness? What are the determinants of fluid responsiveness?
Fluid responsiveness has been defined as a 10-15% increase in cardiac output after a 500 cc bolus fluid challenge. I find this arbitrary definition unhelpful, but I do think that understanding what determines a fluid bolus leading to a preload-responsive state is important.
Figure 1: Frank Starling curve
When giving a fluid bolus, the expectation is that it will increase cardiac preload (by increasing both the stressed volume and mean circulatory filling pressure). Once this condition has been met, the next assumption is that the increase in venous return will lead to a change in stroke volume/cardiac output (venous return = cardiac output). This is the ideal situation! Both ventricles working on the ascending limb of the Frank-Starling curve without any major changes in other determinants of cardiac output (contractility, afterload, diastolic function) Figure 1. Unfortunately, our critically ill patients are not that simple and most times display significant changes in cardiac contractility (e.g., due to acidosis) and afterload (e.g., due to vasoactive agents) that could place them on the flat portion of this curve.
Cardiac Output = Heart Rate x Stroke Volume
Stroke Volume is determined by: contractility, preload, afterload
How do we measure fluid responsiveness?
Despite a great amount of evidence showing that “static” measurements, such as central venous pressure (CVP) are poor predictors of fluid responsiveness, they continue to be widely used. I don’t mean to say that measuring CVPs is useless. After all, perfusion is determined by the pressure gradient between MAP (mean arterial pressure) and CVP, and CVP is a good marker of preload (just NOT of preload responsiveness).
To circumvent this limitation, we can use “dynamic” measurements of heart-lung interactions during mechanical ventilation. Specifically, if changes in intrathoracic pressure under positive pressure ventilation lead to cyclic changes in stroke volume (stroke volume variation or SVV), pulse pressure (pulse pressure variation PVV), or vena cava diameter it indicates that both ventricles are preload-dependent and the patient is fluid responsive. Limitations to these 3 methods include: a) patients need to be mechanically ventilated and without spontaneous breaths (during which changes in intrathoracic pressure become unreliable) b) tidal volume of at least 8 cc/Kg and normal respiratory system compliance (in order to generate significant swings in intrathoracic pressure, tidal volume needs to be on the high end and your lungs/chest wall can’t be stiff).
So you can think about how limited these maneuvers are in the ICU setting. Most intubated patients take spontaneous breaths (unless paralyzed or deeply sedated). If paralyzed for ARDS, they need to be on low-tidal volume ventilation and their lungs are generally pretty stiff.
As you mentioned a maneuver that is unaffected by these limitations is the passive leg raise test (PLR). Importantly, PLR raises preload by shifting venous blood not only from your lower extremities, but mainly from your splanchnic compartment (where ~70% of unstressed volume reservoir lies). This is why in order to adequately perform the maneuver, patients are placed in a semi-recumbent position (rather than horizontal) and the bed is adjusted to 45֯, followed by assessment of cardiac output changes within 60 seconds (NOT blood pressure changes).
PLR is one of the most validated maneuvers for assessment of fluid responsiveness, and as you described, many downstream methods to measure cardiac output have been used, including: a) velocity time integral of the left ventricular outflow tract b) peak velocity of the carotid artery c) changes in end-tidal CO2.
Given the widespread use of critical care echocardiography and their inherent practicality, I think echocardiographic indices are the most useful dynamic parameters to predict fluid responsiveness.
What to expect and my two cents on fluid responsiveness
Given the pace of technology, innovation in hemodynamic monitoring methods will likely improve in the not too distant future. Pocket echo probes and non-invasive wearable sensors measuring cardiac output will make assessment of fluid responsiveness much easier and reliable. Check out:
Michard et al. Intensive Care Medicine (2017) 43:440-442 https://link.springer.com/article/10.1007%2Fs00134-016-4674-z
Vincent et al. Intensive Care Medicine (2018) 44:922-924 https://link.springer.com/article/10.1007%2Fs00134-018-5205-x
For now, I go to the bedside and after a physical exam perform a basic point-of-care ultrasound to assess heart function, look for interstitial lung edema (B lines) and IVC collapsibility before and after a PLR maneuver (if tolerated). Combining information from these maneuvers not only allows you to better assess a patient’s current volume status and likelihood to be fluid responsive (hyperdynamic LV, absent B lines, collapsible IVC), but can also help you identify what type of shock your patient has and if giving fluid could make things worse (RV failure).
When a patient is likely fluid responsive, I give a small/moderate fluid bolus (250-500 cc) then come back to the bedside to repeat my assessment with the tools I feel most comfortable. Then I ask myself: are things getting better? (Improved mental status/blood pressure, increased in urine output and decreased vasoactive agent requirement). And then do it all over again!
Luisa Morales-Nebreda, MD
Fellow, Pulmonary & Critical Care Medicine
Department of Medicine
Northwestern University
How to Cite this Post
[Peer-Reviewed, Web Publication] Cohen B, Wilson D. (2019, Aug 5). Fluid Responsiveness. [NUEM Blog. Expert Commentary by Morales-Nebreda L]. Retrieved from http://www.nuemblog.com/blog/fluid-responsiveness.