Written by: Samantha Stark, MD (NUEM ‘20) Edited by: Steve Chukwulebe, MD (NUEM ‘19) Expert Commentary by: Seth Trueger, MD, MPH
It’s the first few minutes of your shift, and the paramedics roll by your workstation with your first patient, a young woman clutching an inhaler and breathing with every accessory muscle in her body. You direct them to your resuscitation room and they inform you that she has a history of asthma and is having an attack; she’s too exhausted from breathing to verify this, but it seems true. You quickly get her on BiPAP, which mildly improves her work of breathing, but as she becomes drowsy, you obtain a VBG showing a climbing CO2 of 45. You realize that it’s time to intubate this patient, and as you get set up, you collect your thoughts and quickly review everything you’ve heard about intubating asthmatics.
Obstructive Airway Disease
First, remember that asthma is an obstructive airway disease, meaning that there are two main processes to worry about during and after intubation:
Auto PEEP
Hypotension secondary to increased intrathoracic pressure from auto PEEP
*Note: auto PEEP is caused by breath stacking in a patient whose expiration is impaired (such as asthma or COPD) – the ventilator initiates a breath before there’s time for full exhalation, and this leads to progressively more volume retained in the lungs, increasing the risk of barotrauma. This can also lead to increased intrathoracic pressure, in turn decreasing preload to the heart and thus causing hypotension.
How to optimize the intubation:
As you already have this patient on BiPAP, try to preoxygenate as much as possible with this mode of positive pressure
Consider attempting delayed sequence intubation with ketamine
It will maintain the patient’s respiratory drive and may help with BiPAP synchrony and anxiolysis
It serves as a bronchodilator
Use rocuronium for paralysis
It will last longer than succinylcholine, and initially help with vent synchrony
*Note: remember to fully sedate the patient after intubation, as they won’t tell us that they’re not sedated during their prolonged paralysis
Decrease the dead space and resistance of your vent by using the largest endotracheal tube feasible
Frequently reassess the ventilator to ensure that breath stacking is not occurring:
Low respiratory rate to allow for exhalation
Higher tidal volumes of 6-8 cc/kg IBW
Decreased I:E ratio (at least 1:3, may very well need to be longer)
You’ve successfully intubated this patient, and now the lab pages you to let you know that there is a patient in the waiting room with a bicarb of 9. When the patient is wheeled back, his marked tachypnea and work of breathing makes you think he may need to be intubated as well. But he’s so acidotic, and you’re sure you’ve hear something about intubating acidotic people…
Metabolic Acidosis
What you’ve heard is that if you intubate a severely acidotic patient, you’ve killed them. There are two reasons for this:
It’s very difficult to keep up with their minute ventilation
There is a transient increase in pCO2 with paralysis (this is normally inconsequential, but in the decompensating acidotic patient, can lead to cardiovascular collapse)
How to optimize the intubation:
Optimize cardiovascular status as much as possible beforehand
Bolus fluids
Consider starting pressors pre-intubation, or having push-dose phenylephrine or epinephrine on hand during intubation
Match the patient’s minute ventilation
Ensure adequate pre-oxygenation, using NIPPV
However, even if oxygenation is not an issue, BiPAP should be used to assess the minute ventilation the patient is maintaining on their own, to help determine what is needed post intubation
Using delayed sequence technique with ketamine as the induction agent and a short acting paralytic like succinylcholine could theoretically help to avoid apnea as much as possible
Once intubated, the patient’s pre-intubation minute ventilation (respiratory rate and tidal volume) MUST be matched on the ventilator
Don’t be surprised to see higher tidal volumes of 8 cc/kg IBW
As you’re sitting down to catch up on notes, a nurse gets your attention to let you know that there is an altered, febrile, tachycardic patient with a pressure of 65/40 tucked away in a bed at the back of the ED that you should probably see right away. As it turns out, this patient needs to be intubated as well.
Shock
As mentioned above, increased intrathoracic pressure from PPV results in decreased venous return to the heart, leading to decreased preload. This obviously has the potential to be quite detrimental to a patient with shock.
How to optimize the intubation:
Optimize cardiovascular status as much as possible beforehand
Fluid resuscitation and vasopressors started prior to intubation
Have push dose pressors available at the bedside should they be needed
Induction agents:
Avoid propofol as it has a propensity to cause hypotension
Use etomidate or ketamine
Ketamine has been shown to be more hemodynamically stable than etomidate
Also, the body should prioritize cerebrovascular blood flow in shock, therefore if etomidate is used, consider decreasing the dose to minimize hemodynamic effects
At this point, you’re too tired to write any notes, so you decide to sit down and, given how your shift has been going so far, do some reading about patients that are dangerous to intubate or difficult to manage on the vent. The first topic you come across is pulmonary hypertension.
Pulmonary Hypertension
Mechanical ventilation is dangerous in these patients due to their inability to tolerate decreased preload, increased afterload, or really any alteration in their tenuous hemodynamics. Unfortunately, in patients with pulmonary hypertension but also systemic hypotension, IV fluids can over-distend the right ventricle and make things worse. There’s not a super reliable way to tell if these patients will be fluid responsive or not; most would suggest a small fluid bolus challenge to see how they respond. There may or may not be time for this prior to intubation, but if there is time, it’s probably worth a try.
How to optimize the intubation:
Can consider pre-medication with fentanyl:
Thought to blunt the hypertensive response to laryngoscopy, similar to head-injured patients
In theory, this prevents increased afterload in the pulmonary vasculature
Induction agent:
Consider etomidate
Theoretically should have less of an effect on preload than propofol
Additionally, less of an effect on afterload than ketamine
Ventilator settings:
Closely monitor plateau pressures to keep them less than 30 cm H2O, to avoid drops in preload due to increased intrathoracic pressure
Consider placing an arterial line for frequent ABG checks
Both hypercapnia and hypoxia can cause vasoconstriction (increasing afterload in the pulmonary vasculature)
Two days later, while you’re following up on some of your prior patients, you note that the patient in septic shock that you intubated a couple of days ago now has ARDS, and it seems that the inpatient team is having some difficulty managing her on the vent.
ARDS
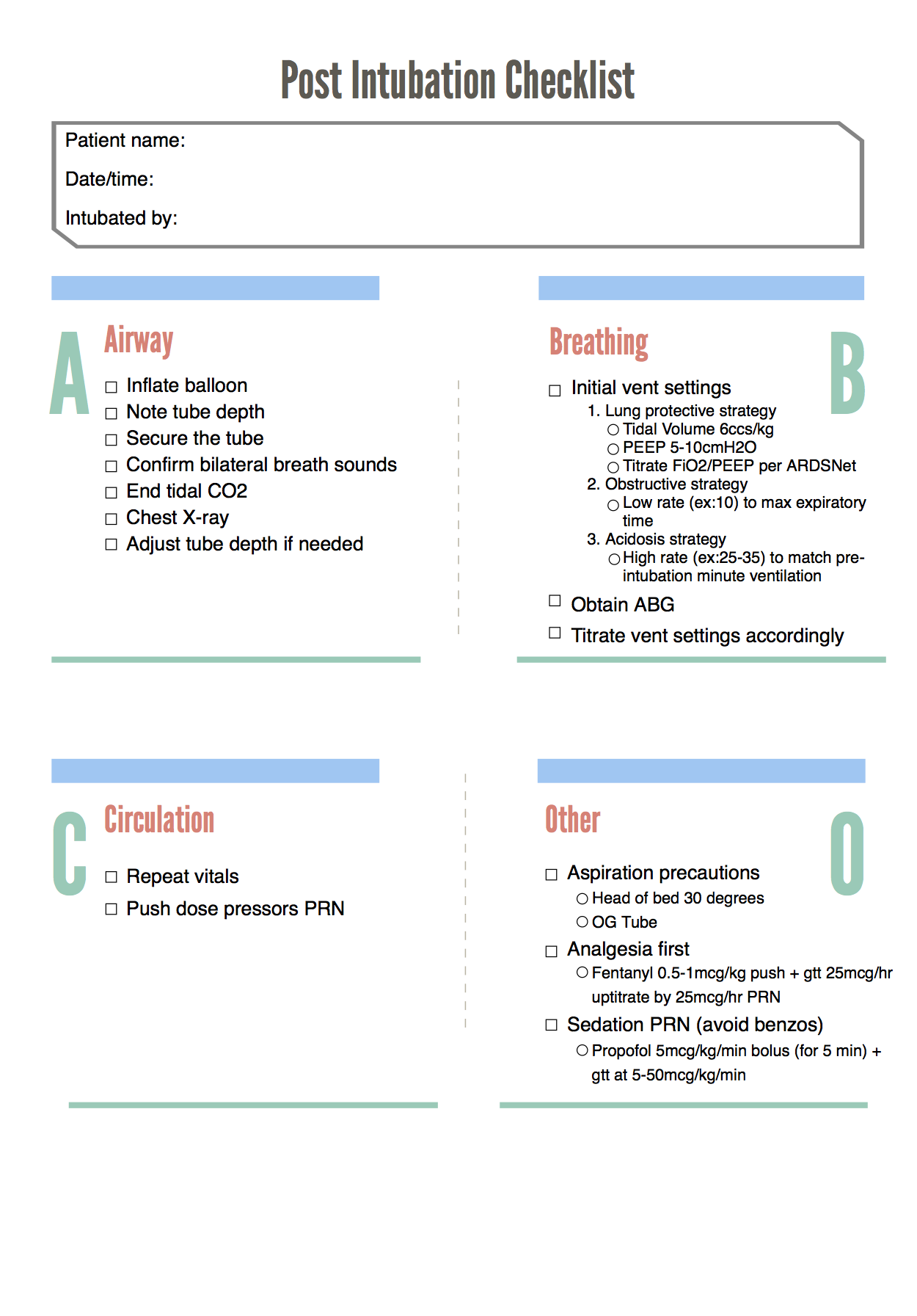
While this is an area of active research and there are different strategies and methods for helping to improve these patients’ oxygenation, the main thing to remember from the perspective of managing the ventilator is the lung protective strategy:
Tidal volume 6 cc/kg IBW
Plateau pressure less than 30 cm H2O
Minimum PEEP of 5 cm H2O (and remember that these patients may often need significantly higher PEEP)
Expert Commentary
Thank you for this review of intubating sick patients - intubating complex physiology is arguably one of the most dangerous things we can do, but there are some straightforward, concrete steps we can take to do it as safely as possible.
For me, the first step is to consider every ED intubation potentially dangerous. Maximize resuscitation (IV fluids; pressors if needed, always ready) and optimize preoxygenation to provide the biggest possible safety net. It’s much more CBA than ABC.
Every patient we intubate in the ED has potential to crump: the sympatholysis from sedation will reduce endogenous catecholamines, and the switch to positive pressure ventilation impairs preload.
Every intubated patient needs post-intubation sedation. I generally default to a fentanyl drip and modify from there (eg add propofol if BP tolerates; add ketamine if not). Do not remove sedation for hypotension; do not use pain as a pressor. That is torture and it is bad. Sedate the patient adequately and if that means more resuscitation (fluid, blood, pressors, etc) then do that too. Do not torture patients to maintain BP.
The easiest tactic to ensure post-intubation sedation is to think of RSI as 3 medications: NMBA, induction agent, and post-intubation sedative. I should not be surprised that I will need post-intubation sedation shortly after intubation.
Perhaps the biggest lesson in ARDS management and prevention in recent years is that nearly everyone potentially benefits from lung protective ventilation, i.e. 6 ml/kg *ideal* body weight. I’ve changed my default tidal volume to 400-450ml (it was 550-600 when I was in med school). Otherwise, ventilation (minute ventilation, or CO2 management) is all about adjusting respiratory rate (my default is 16-18, not 12) as the patient’s height usually does not change in the ED.
Special situations: asthma patients don’t have a big enough tube to exhale properly. Pay special attention, make sure they have sufficient time to exhale (and they may the one group that may benefit from *not* being on 6 ml/kg IBW. Perhaps even more importantly, unlike many other situations, intubation does not fix asthma; it makes it even harder to manage, as even the largest ET tubes are, by definition, smaller than the patient’s natural airway. Maximize NIV and other management options (eg epinephrine) if at all possible.
Acidosis is tough and the key is maximizing ventilation before and after intubation. These patients may need absurd-seeming respiratory rates and regardless of how hypercarbic they are, acidosis does not make patients taller so there is no reason to adjust tidal volume.
Pulmonary hypertension is complex and scary. Prepare beforehand, and work with your intensivists and other relevant specialists.
The most important part of airway management is preparation – not just in the ED, but learning as much as I can beforehand.
Seth Trueger, MD, MPH
Assistant Professor of Emergency Medicine
Department of Emergency Medicine
Northwestern University expert commentator
How To Cite This Post:
[Peer-Reviewed, Web Publication] Stark, S. Chukwulebe, S. (2020, Oct 5). Physiologically Difficult Intubations. [NUEM Blog. Expert Commentary by Trueger, S]. Retrieved from http://www.nuemblog.com/blog/physiologically-difficult-intubations
Other Posts You May Enjoy
References
Ebert TJ, Muzi M, Berens R. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725-33.
Van Berkel MA, Exline MC, Cape KM, et al. Increased incidence of clinical hypotension with etomidate compared to ketamine for intubation in septic patients: a propensity matched analysis. Journal of Critical Care. 2017;38:209-214.
Dalabih M, Rischard F, Mosier JM. What’s new: the management of acute right ventricular decompensation of chronic pulmonary hypertension. Intensive Care Med. 2014;40(12):1930-3.
Hemmingsen C, Nielson PC, Odorico J. Ketamine in the treatment of bronchospasm during mechanical ventilation. Am J emerg Med. July 1994;12(4):417-420.
Eames WO, Rooke GA, Wu RS, Bishop MJ. Comparison of the effects of etomidate, propofol, and thiopental on respiratory resistance after tracheal intubation. Anesthesiology. June 1996;84(6):1307-11.
Gragossian A, Asp A, Hamilton R. High Risk Post Intubation Patients. www.emdocs.net/ high-risk-post-intubation-patients/ June 2017
The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes fo acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
NIH NHLBI ARDS Clinical Network. Mechanical Ventilation Protocol Summary. www.ardsnet.org/files/ventilator_protocol_2008-07.pdf
Marino, Paul L. 2009. The Little ICU Book. Wolters Kluwer Health. Philadelphia, PA.
Arbo, John E. 2015. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Wolters Kluwer Health. Philadelphia, PA.