Written by: Michael Tandlich, MD (NUEM ‘24) Edited by: Chloe Renshaw, MD (NUEM ‘22)
Expert Commentary by: Justin Seltzer, MD (NUEM ‘21)
Expert Commentary
An excellent post by Drs. Tandlich and Renshaw. Marine envenomations are common problems around the world. Like with land-based envenomations, the venomous organisms of note vary with geography; jellyfish encountered in Australia are different from those encountered in Florida, for example. As a result, we will focus on major envenomations in the United States.
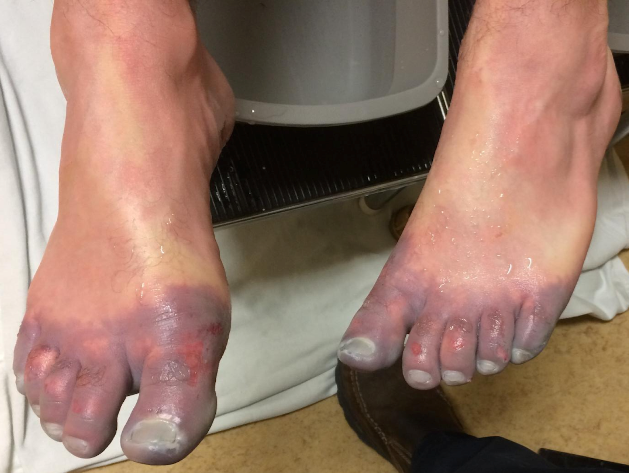
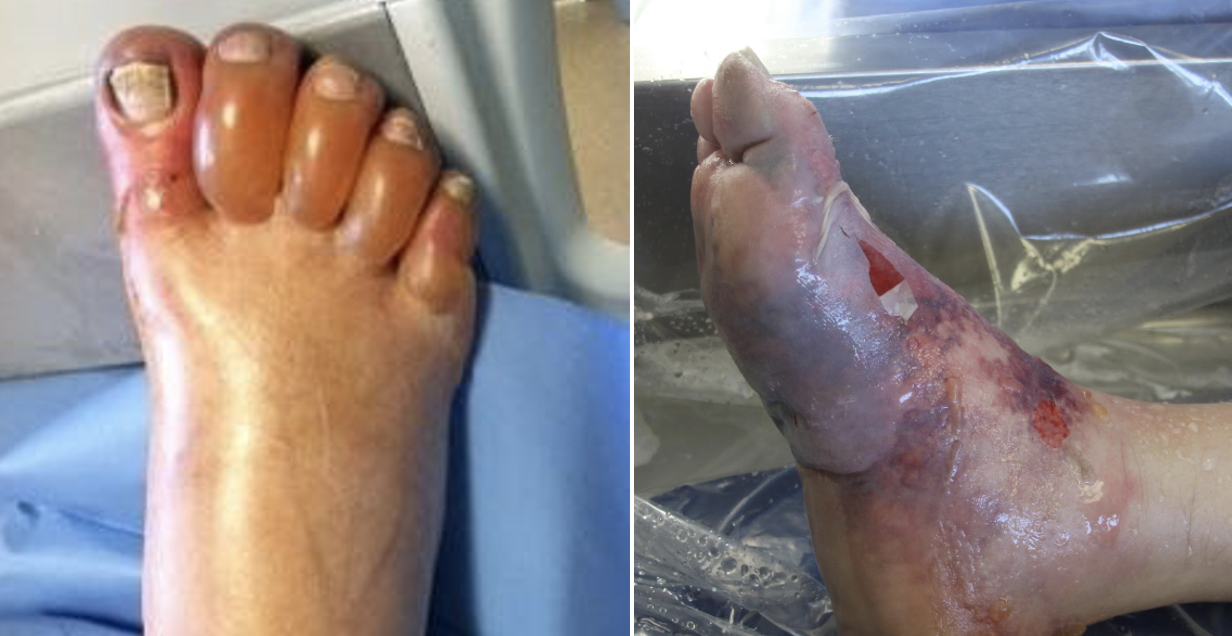
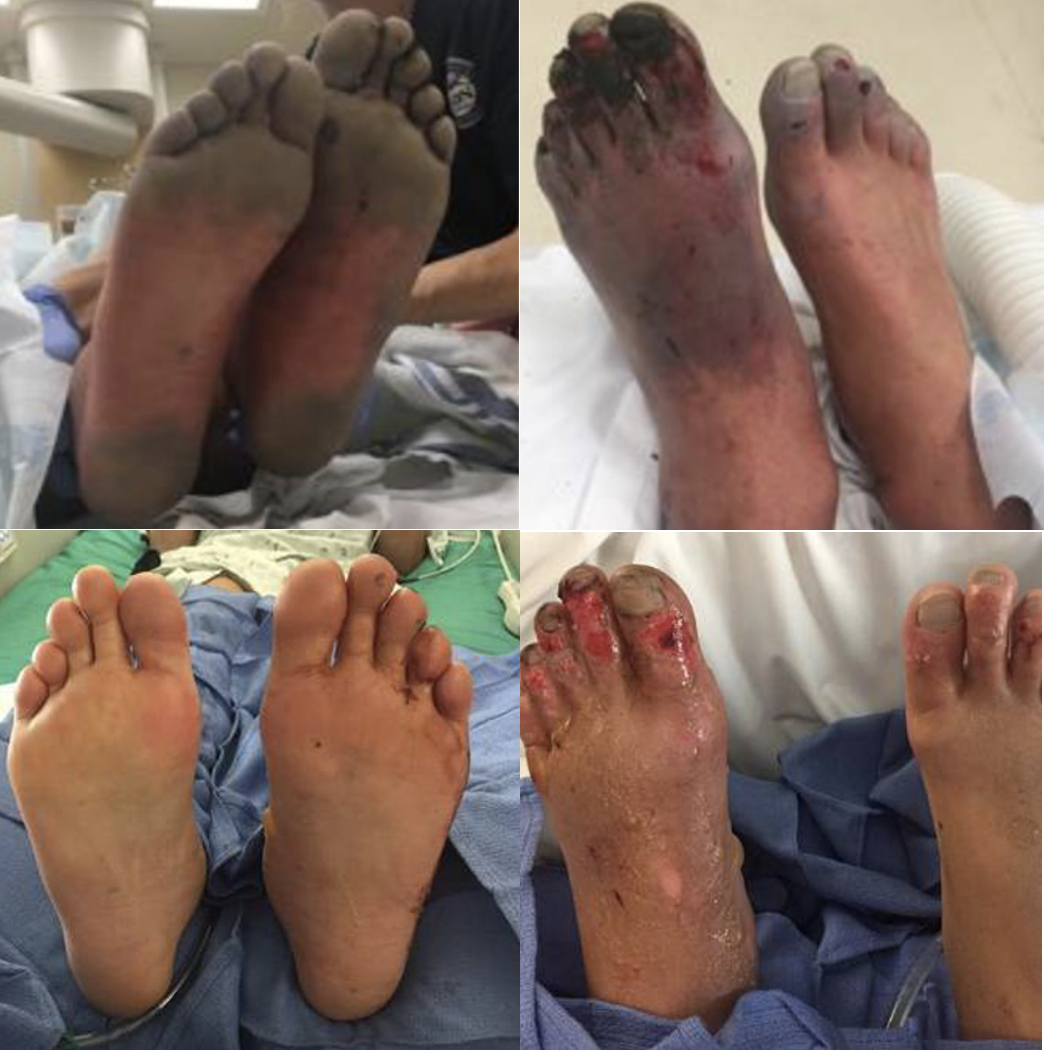
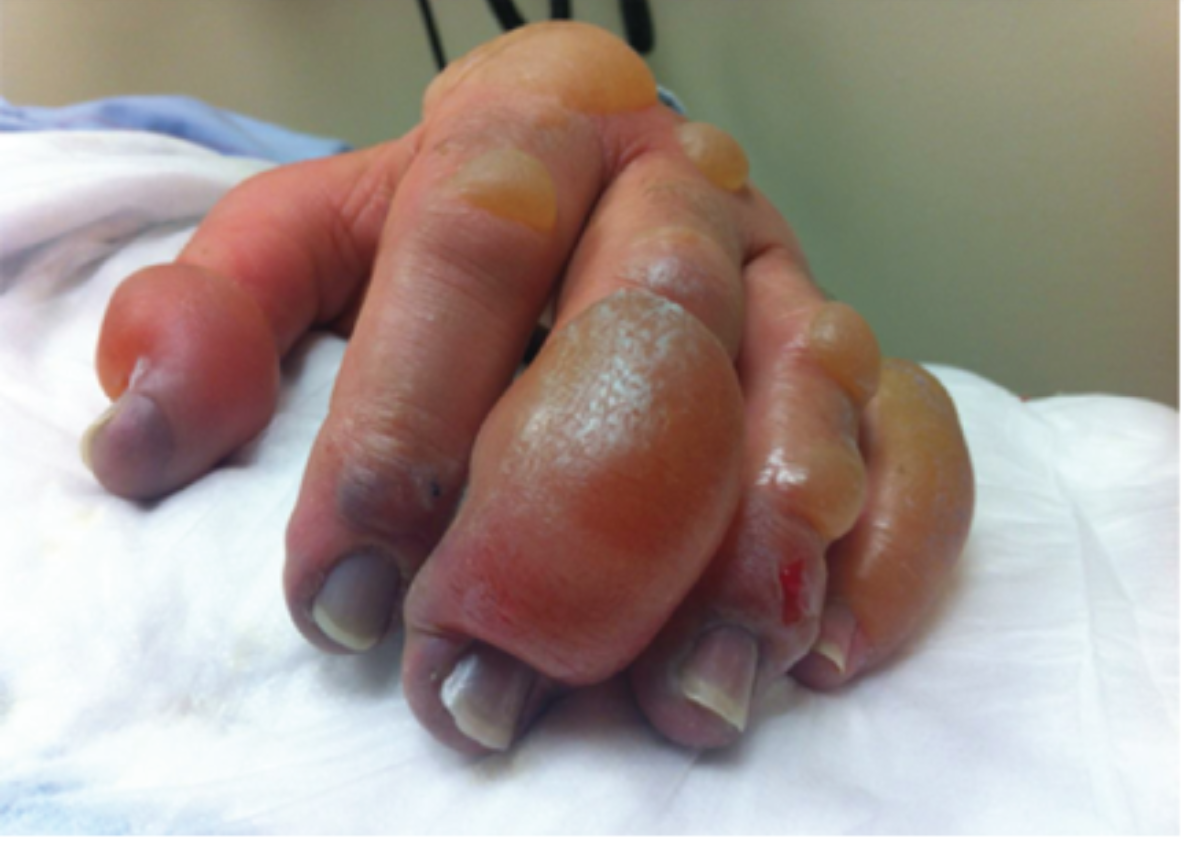
The invertebrates account for a large but ultimately unknown number of envenomations. Cnidaria includes jellyfish, hydrozoa, anemones, and fire coral. A majority of stings from this group result in painful dermatitis; tentacles create a “whip-like” pattern on the skin, whereas fire coral creates localized skin wheals. The sea nettle and Portuguese man-of-war are of greatest interest, given their potential to cause severe systemic symptoms. Box jellyfish are rare in US coastal waters but produce a life-threatening toxicity.
Initial treatment is somewhat controversial. Many resources advocate for the use of seawater for the initial decontamination, given concern for vinegar triggering nematocyst release in some species common to US waters. However, further research is needed to determine which is best. At this time, seawater is recommended for empiric decontamination in the US unless a box jellyfish is strongly suspected, in which case vinegar is appropriate (a very rare circumstance). Systemically ill box jellyfish envenomations should be treated with pain and blood pressure control. The antivenom is not readily available in the US and is unlikely to be beneficial in the time course it would take to obtain it.
Echinodermata, which includes sea urchins, have mild venom on their spines that can cause local tissue irritation and pain. There are reports of severe envenomations with systemic symptoms, but this is ultimately quite rare. These injuries respond well to hot water immersion. Imaging and local wound exploration for retained spines are recommended. Soaking the wound in vinegar may help dissolve superficial spines.
Of the vertebrates, stingrays and spiny fish are of primary concern.
Stingrays stings are common and can cause serious penetrating trauma but envenomation mainly produces localized pain and swelling. The venom is heat-labile, so significant pain relief can be achieved with hot water immersion. Stingrays stings have the potential for both retained stinger and wound infections; evaluation for retained stinger with radiographs and local wound exploration is recommended along with prophylactic antibiotics.
Spinyfish, in particular stonefish, lionfish, and scorpionfish, have venom located in their spines. Stonefish have the most potent venom of any known fish. Lionfish are not native to the US but have become an invasive species. Human contact with these fish occurs both in the wild and in aquariums. These fish also have heat-labile venom susceptible to hot water immersion. However, systemically ill stonefish envenomations should receive the antivenom as this envenomation can be life-threatening; the antivenom will likely work against other spiny fish too, however, these other envenomations are usually much less severe and rarely require more than hot water immersion and supportive care.
So key learning points:
Most marine envenomations involve heat-labile venom. Hot water immersion is likely to help reduce local symptoms.
Systemic illness is rare but some marine envenomations can produce life-threatening toxicity. Be very wary of a systemically ill envenomation and try to figure out the source due to the limited availability of antivenoms.
Prophylactic antibiotics are recommended for stingray stings as they tend to get infected but otherwise are generally not necessary in most populations. Good wound care, evaluation for retained foreign bodies, and tetanus prophylaxis are the mainstays.
For further information, see this review article
Justin Seltzer, MD
UCSD Health Toxicology Fellow
Emergency Physician, UCSD Health
How To Cite This Post:
[Peer-Reviewed, Web Publication] Tandlich, M. Renshaw, C. (2022, Mar 7). Marine Envenomations. [NUEM Blog. Expert Commentary by Seltzer, J]. Retrieved from http://www.nuemblog.com/blog/marine-envenomations