Written by: Rafael Lima, MD (NUEM ‘23) Edited by: Laurie Aluce, MD (NUEM ‘21)
Expert Commentary by: Zachary Schmitz (NUEM ‘21)
Methanol Toxicity
Methanol itself is not toxic to the body. Methanol’s metabolite, formic acid, causes toxicity at serum levels greater than 20mg/dl [1].
Clinical Findings of Methanol Poisoning
CNS sedation
Seizures
Rapid, Deep Breathing
Hypotension
Ocular findings:
Blindness
Afferent pupillary defect
Optic disk hyperemia
Mydriasis
Ethylene Glycol Toxicity
Similarly, the toxic metabolites of ethylene glycol cause end-organ damage at levels greater than 20mg/dl. The most notable toxic metabolites are glycolic acid and oxalic acid.” [1] .
Clinical Findings of Ethylene Glycol Poisoning
CNS sedation
Seizures
Cranial nerve palsies
Rapid, deep breathing
Hypotension
Hypocalcemia (can result in tetany)
Renal findings:
Oliguria
Acute renal failure
Flank pain
Hematuria
Oxalate crystals in the urine under fluorescence
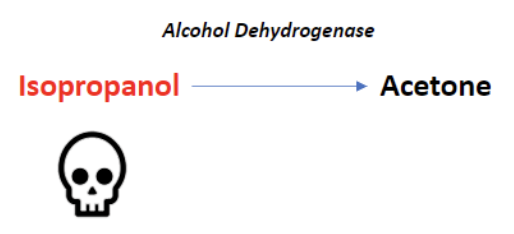
Isopropyl Alcohol Toxicity
Found in hand sanitizers and disinfectants, isopropyl alcohol is a less common source of alcohol poisoning. The parent molecule does exhibit toxic effects here, unlike methanol and ethylene glycol. If untreated, the lethal dose is between 4-8 g/kg [2].
Alcohol dehydrogenase metabolizes isopropyl alcohol into acetone. Because acetone is a ketone, and ketones are not oxidized into carboxylic acids, isopropyl alcohol poisoning does not result in anion gap metabolic acidosis.
Clinical Findings of Isopropyl Alcohol Poisoning
CNS sedation
Disconjugate gaze
Fruity breath odor
Hypotension
Hematemesis
Pulmonary edema
Plasma Osmolal Gap
One of the most reliable laboratory markers of toxic alcohol poisoning is a large osmolal gap. The osmolal gap is defined as the difference between the measured serum osmolality and the calculated, or expected, plasma osmalality:
OSMOLAR GAP = Measured plasma osmolality – calculated/expected plasma osmolality
The common equation for calculating the expected plasma osmolality is listed below [3]. Of note, there are other formulas with slight variations. Using an online calculator can be helpful.
Expected Serum Osmolality=2[Na]+BUN/2.8+Glucose/18
A gap < 10 is considered normal. Any elevation above 10 should raise the clinician’s suspicion of toxic alcohol ingestion.
Note: this tool is not helpful in late presentations as the metabolized forms of the different alcohols do not contribute to the osmolal gap. The calculated gap will be falsely low in late-stage poisoning.
Treatment of Toxic Alcohol Ingestions
Consult your medical toxicologist or poison control center if toxic alcohol ingestion is suspected.
The national poison control center hotline telephone number is 1(800)-222-1222.
Fomepizole
Fomepizole should be used only for methanol and ethylene glycol ingestions. It is not indicated for isopropyl alcohol intoxications [4]. It is an inhibitor of alcohol dehydrogenase (ADH). Evidence shows that it is a superior antidote to ethanol [5].
Loading dose 15 mg/kg IV
Then 10 mg/kg every 12 hours
Continue until blood pH is normal and serum alcohol concentration is less than 20 mg/dL in the presence of retinal or renal injury.
Ethanol
Ethanol works as a competitive inhibitor of ADH, having a higher affinity for the enzyme compared to the other alcohols. Ethanol was used historically before the effects of fomepizole were studied. Fomepizole is now the preferred treatment because the administration of ethanol is more difficult, ethanol causes sedation, and titration of the therapy is challenging in co-ingestions [6]. If ethanol must be used, the preferred route is IV and the studied therapeutic target level is 100 mg/dL [7].
Supplemental Therapy
Methanol poisoning patients should also receive folic acid (50mg IV every 6 hours) [7].
Ethylene glycol poisoning patients should also receive thiamine (100mg IV) and pyridoxine (50mg IV) [8].
Hemodialysis
Consult your nephrologist early if you are considering hemodialysis. Renal replacement therapy should be considered in the following situations [9]:
Anion gap metabolic acidosis with known toxic alcohol ingestion
End-organ damage
Renal failure
Vision changes
Unexplained anion gap metabolic acidosis with elevated osmolal gap in suspected toxic alcohol ingestion
References
1. Liesivuori, J. and H. Savolainen, Methanol and formic acid toxicity: biochemical mechanisms. Pharmacol Toxicol, 1991. 69(3): p. 157-63.
2. Slaughter, R.J., et al., Isopropanol poisoning. Clin Toxicol (Phila), 2014. 52(5): p. 470-8.
3. Bhagat, C.I., et al., Calculated vs measured plasma osmolalities revisited. Clin Chem, 1984. 30(10): p. 1703-5.
4. Su, M., R.S. Hoffman, and L.S. Nelson, Error in an emergency medicine textbook: isopropyl alcohol toxicity. Acad Emerg Med, 2002. 9(2): p. 175.
5. McMartin, K., D. Jacobsen, and K.E. Hovda, Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol, 2016. 81(3): p. 505-15.
6. Zakharov, S., et al., Fomepizole versus ethanol in the treatment of acute methanol poisoning: Comparison of clinical effectiveness in a mass poisoning outbreak. Clin Toxicol (Phila), 2015. 53(8): p. 797-806.
7. Barceloux, D.G., et al., American Academy of Clinical Toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol Clin Toxicol, 2002. 40(4): p. 415-46.
8. Ghosh, A. and R. Boyd, Leucovorin (calcium folinate) in "antifreeze" poisoning. Emerg Med J, 2003. 20(5): p. 466.
9. Moreau, C.L., et al., Glycolate kinetics and hemodialysis clearance in ethylene glycol poisoning. META Study Group. J Toxicol Clin Toxicol, 1998. 36(7): p. 659-66.
Expert Commentary
Thank you for this great review of a difficult subject! The combination of a lack of quick, confirmatory testing with delayed onset of symptoms makes toxic alcohol poisoning an incredibly difficult diagnosis to make. Additionally, even small ingestion can lead to major complications. For example, if a typical four-year-old (19kg) child drank windshield washer fluid that contained 50% methanol (a fairly standard formulation), it would take only 5.7 mL to potentially produce a methanol serum concentration of 25 mg/dL. Given the average 4-year-old’s mouthful is 8.9 mL, you can run into trouble quickly.[1]
We frequently see misuse or misunderstanding of osmol and anion gaps in diagnosing toxic alcohol ingestion when history is unclear. First, although a normal osmol gap is generally less than 10, baseline osmol gaps range from -10 to +14.[2] Therefore, a gap of 16 may represent a true gap of +2 in one person and +26 in another. Second, ethanol must be included in the osmol gap equation. An ethanol concentration of 200 mg/dL would increase your osmol gap by 43.5. Third, given metabolism over time, all values included in an anion gap calculation need to be drawn off of the same blood sample.
These considerations make finding the diagnosis even more complicated, but there are a few things that can help you out. First, an osmol gap > 50 is highly concerning for toxic alcohol. Second, an ethanol concentration > 100 mg/dL is sufficient to block ADH, meaning that few toxic metabolites from methanol or ethylene glycol could be made.[3] This means that an anion gap present with an ethanol > 100 mg/dL is not from toxic alcohol (unless the patient drank the ethanol after the toxic alcohol, which is very rare). Third, sequential values over time can be helpful. Metabolism of toxic alcohols should lead to a decreased osmol gap and increased anion gap over time. Proper use of the osmol and anion gap can help identify patients at high risk for morbidity and mortality while decreasing unnecessary administration of fomepizole, which typically costs thousands of dollars.
References
Ratnapalan S, Potylitsina Y, Tan LH, Roifman M, Koren G. Measuring a toddler's mouthful: toxicologic considerations. Journal of Pediatrics. 2003 Jun;142(6):729-30. doi: 10.1067/mpd.2003.216
Hoffman RS, Smilkstein MJ, Howland MA, Goldfrank LR. Osmol gaps revisited: normal values and limitations. J Toxicol Clin Toxicol. 1993;31(1):81-93. doi: 10.3109/15563659309000375.
Jacobsen D, McMartin KE. Methanol and ethylene glycol poisonings: mechanism of toxicity, clinical course, diagnosis and treatment. Med Toxicol. 1986;1:309-334.
Zachary Schmitz, MD
Toxicology Fellow
Ronald O. Perelman Department of Emergency Medicine
NYU Langone Health
How To Cite This Post:
[Peer-Reviewed, Web Publication] Lima, R. Aluce, L. (2022, Jan 24). Toxic Alcohols. [NUEM Blog. Expert Commentary by Schmitz, Z]. Retrieved from http://www.nuemblog.com/blog/toxic-alcohols